half life formula chemistry
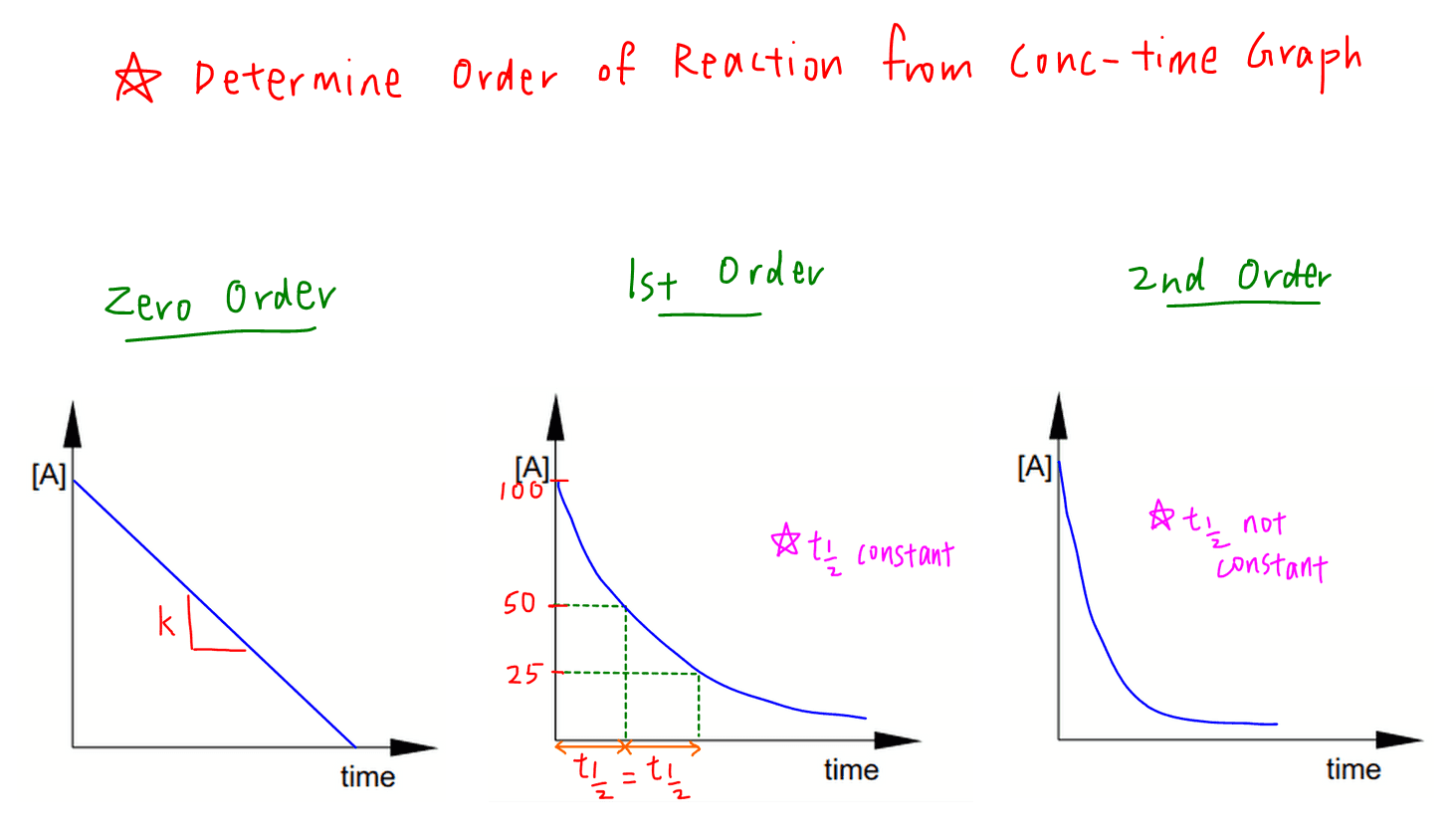
The mathematical expression that can be employed to determine the half-life for a zero-order reaction is t12 R 02k. The equations are given above.
Learn Chemistry Tutorials Kinetics Tutorial
We know that at the half-life time eqt_12 eq the concentration of the reactant will.

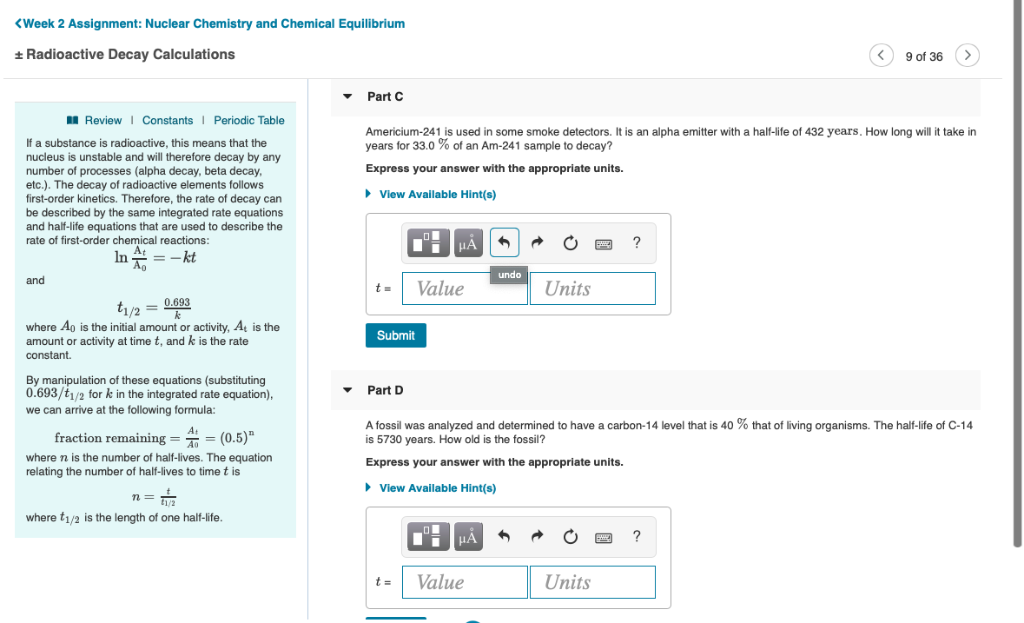
. Using the concentration-time equation for a second-order reaction we can solve for half-life. The half-life formula for various reactions is given below. Given that the half-life of Pd is 4 days calculate the initial mass of the sample.
Half-life a useful concept if its value does not depend on how much. The calculator will automatically compute half-life t 1 2 using equation 2 above. Half life formula- The time taken for half of reactions to complete or the time at which the concentration of the reactant is reduced to half of its original value is called the half life period.
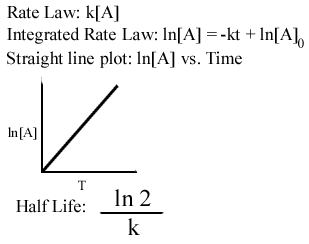
N 0 is the. This becomes evident when we rearrange the integrated rate law for a first-order. So the value of t can be found or determined using half life equation.
The half-life formula for chemistry is T ln2lambda. The half-life formula for a reaction depends upon the order of a reaction. Rearranging the equation used in example 2 we.
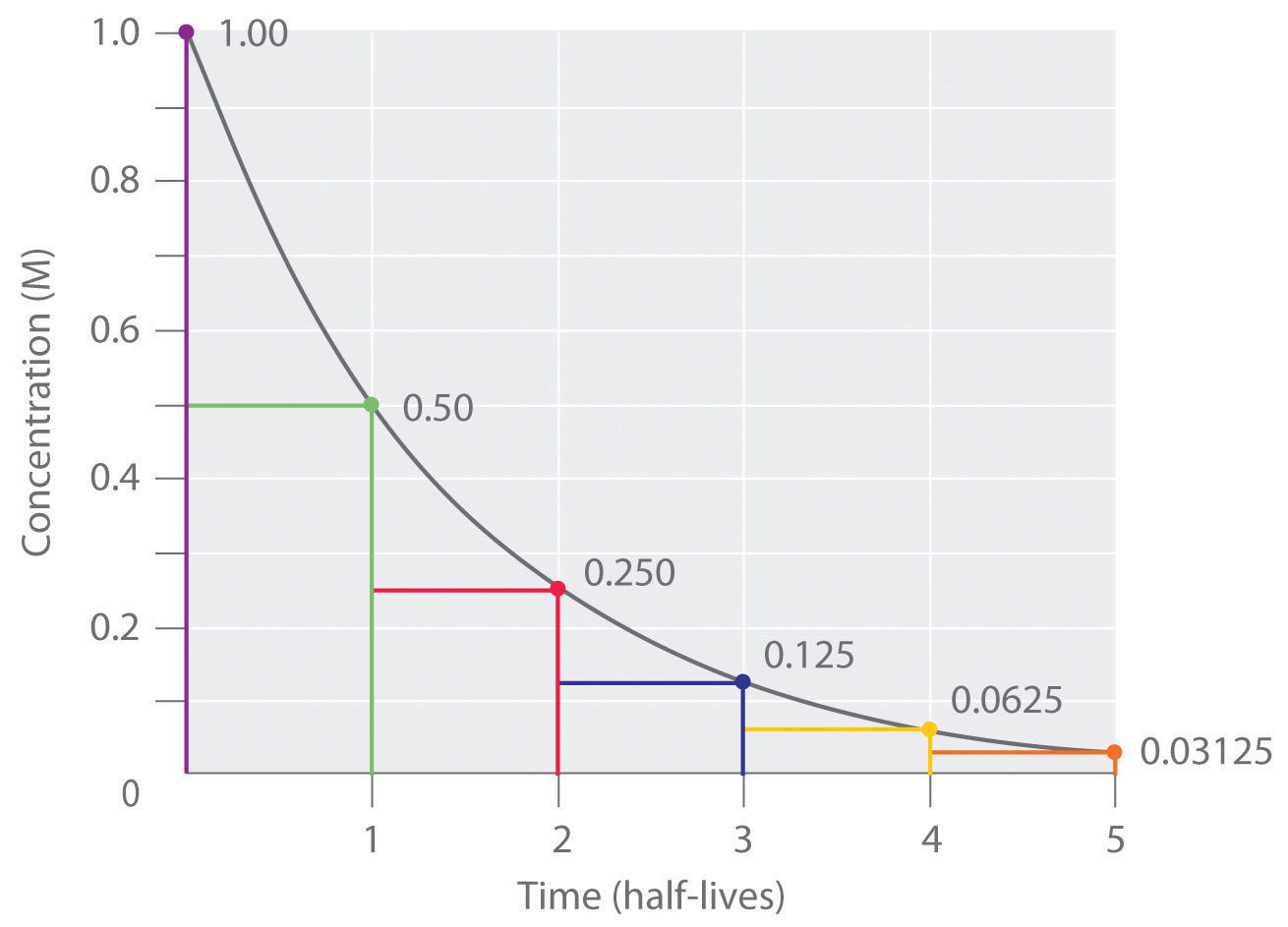
So if you go back after a half-life half of the atoms will now be nitrogen. So now you have after one half-life-- So lets ignore this. Substitute this information into the equation for the half life of a reaction with this order and solve for t ½.
N t N 0 05 t n. By using the following decay formula the number of unstable nuclei in a radioactive element left after t can be calculated. All 10 grams were carbon.
For a first zero order. For a zero-order reaction the half-life equation is given as. We are required to find N o when we have N t and t.
So we started with this. We can determine that the order of a decay reaction is 1 by looking at the. In nuclear reactions this time period.
The second scenario is when you have the percentage of the radioactive material remaining and the total. Converting a Half Life to a Rate Constant. Find the value of the decay constant of a radioactive substance having a half-life of 004 seconds.
In order to calculate the half-life of a chemical specie integrated half-life equations are used according to the order of reactions. N t N_0 times 05 tT N t N 0. Half-Life is the required time for a sample of radioactive material to reach half the mass of the original sample.
What is a simple definition of. The half-life of a first-order reaction is independent of the concentration of the reactants. Given half life of the substance is t1 2 t 1 2 004.
N t is the remaining quantity of a substance after time t has elapsed. Half-life Half-life thalf is defined as the amount of time required for the amount of a substance to be reduced by 50. T is the half-life and lambda is the decay constant which is specific to each chemical.
Solved Week 2 Assignment Nuclear Chemistry And Chemical Chegg Com
Half Lives And Radioactive Decay Kinetics

Ap Chemistry Q3b1 My Ap Chemistry Blog

Half Life Of A First Order Reaction Video Khan Academy

How To Plot A Half Life Graph Chemistry Study Com

Nuclear Chemistry Half Life Transmutation 6 Practice Problems With Answer Key

C 3 Calculating The Decay Constant Sl Youtube

5 Ways To Calculate Half Life Wikihow

Half Life Formula And Radioactive Decay Reactions Medium

Radioactive Decay Model Math And Chemistry Science Activity Exploratorium Teacher Institute Project
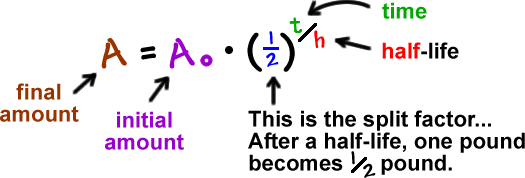
Exponentials Logarithms Cool Math Algebra Help Lessons Radioactive Decay And Decibel Levels
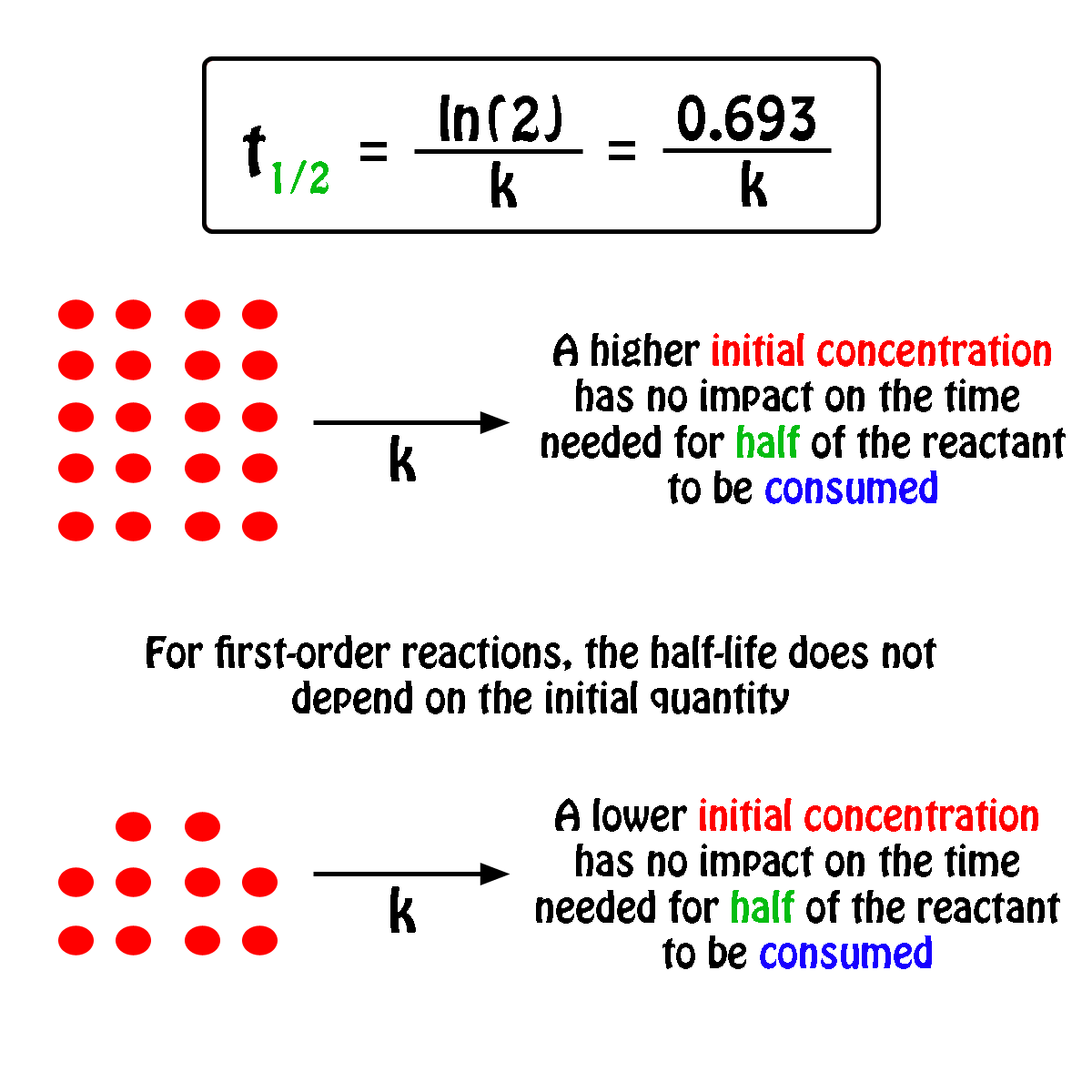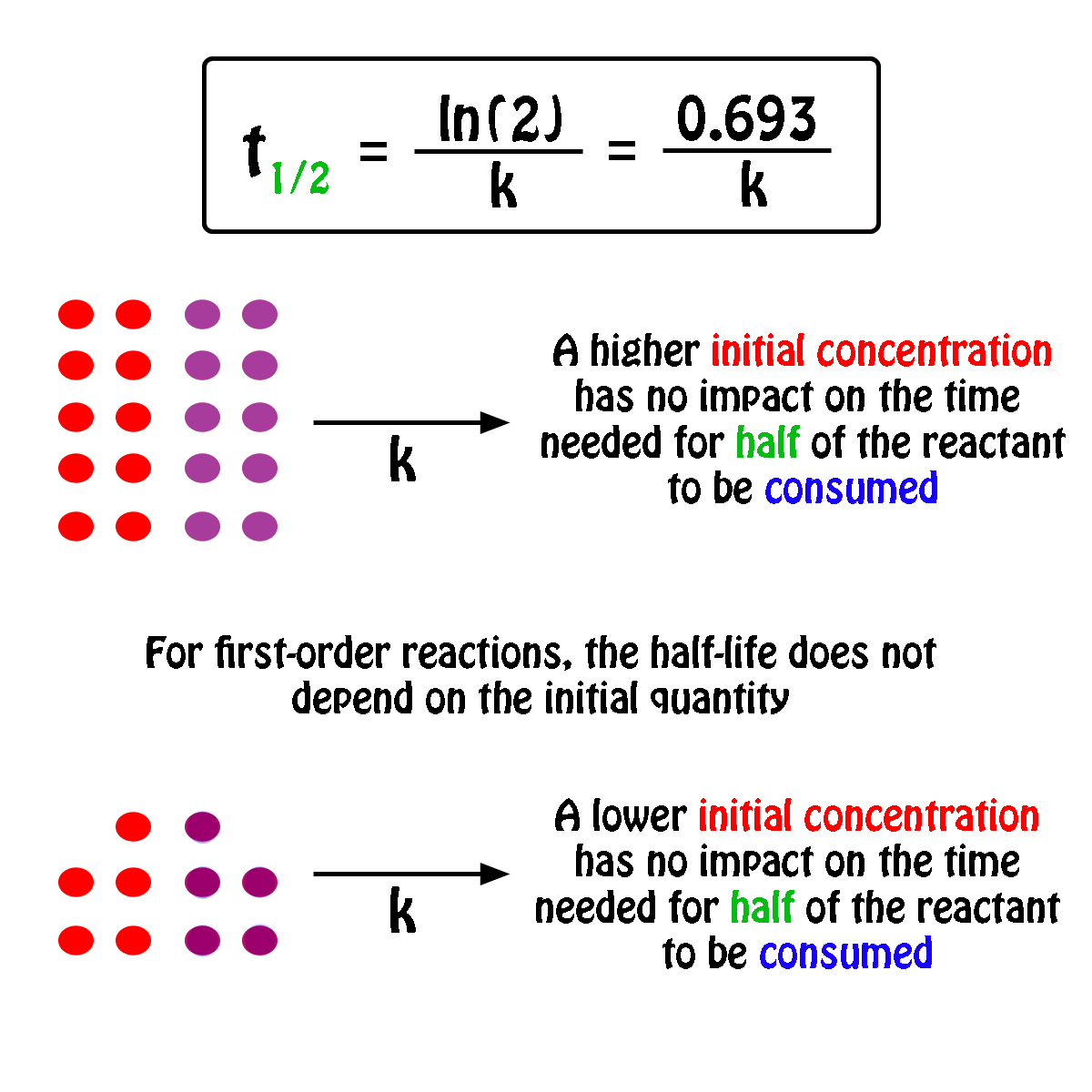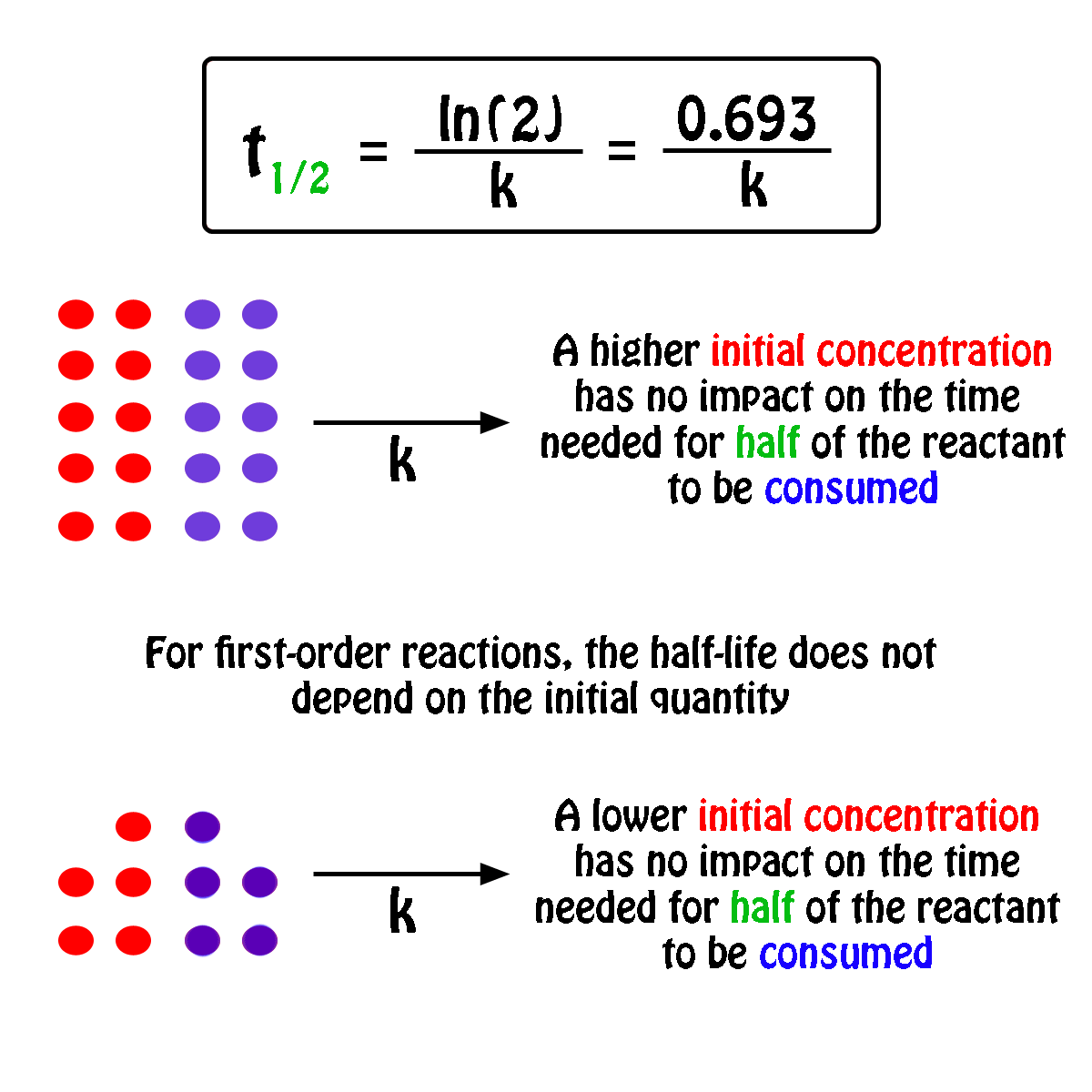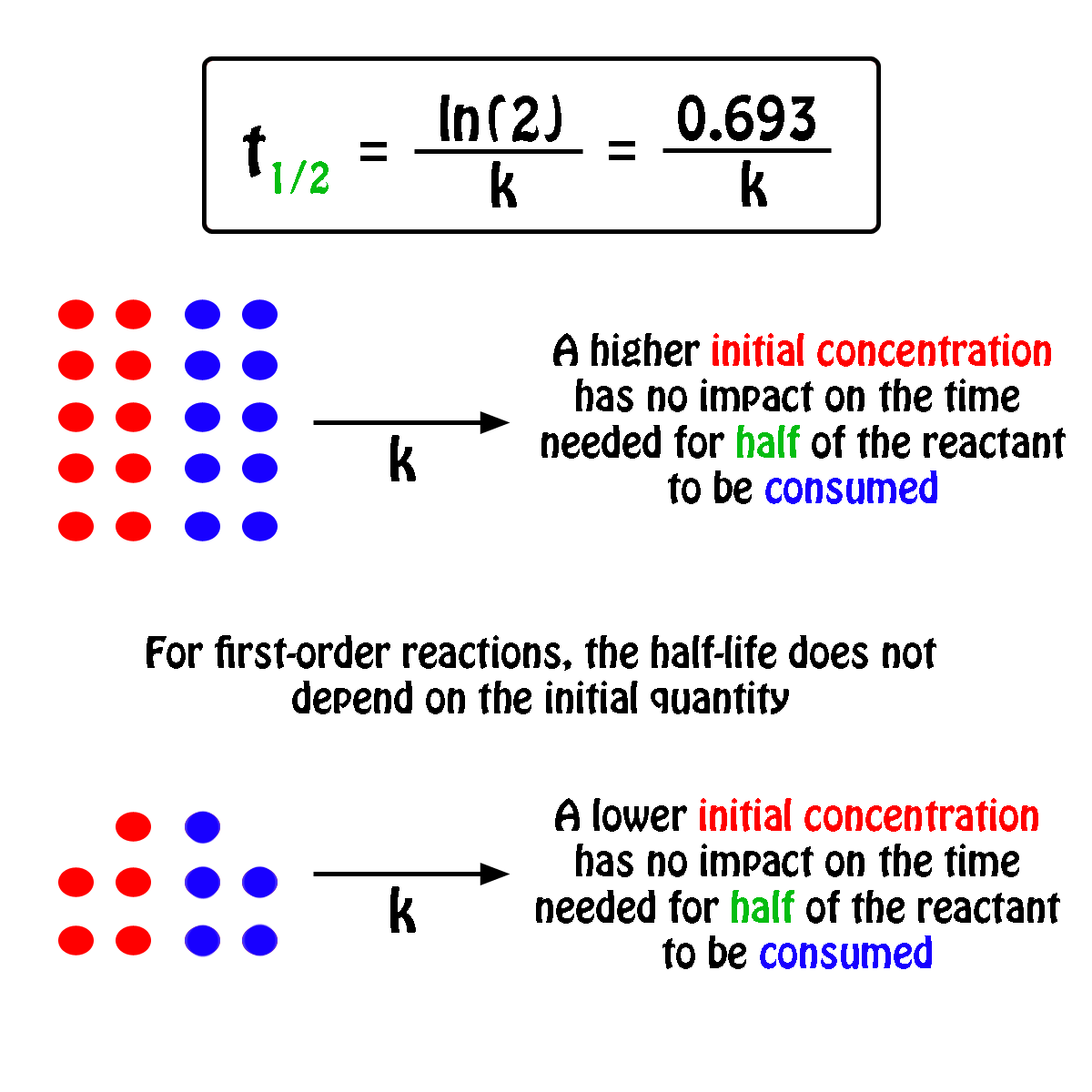
What Is The Half Life Of A First Order Reaction With A Rate Constant Of 7 80xx10 4 S 1 Socratic

Half Life Chemistry Problems Nuclear Radioactive Decay Calculations Practice Examples Youtube

Kinetics Of Radioactive Decay Video Khan Academy
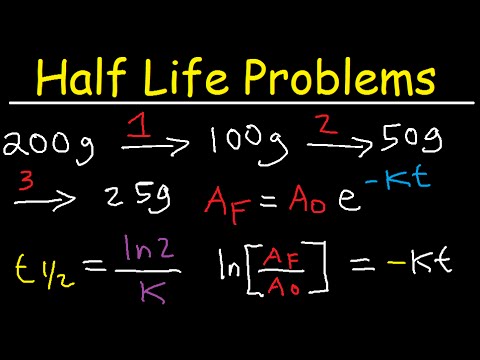
Half Life Chemistry Problems Nuclear Radioactive Decay Calculations Practice Examples Youtube
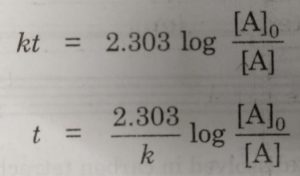
Half Life Period Of A Reaction Chemical Kinetics Chemistry Class 12